CAR-T THERAPY FOR RELAPSED AND REFRACTORY MULTIPLE MYELOMA
CAR-T=chimeric antigen receptor T-cell.
CARVYKTI® was compared to patients receiving: daratumamab, pomalidomide, and dexamethasone or bortezomib, pomalidomide, and dexamethasone

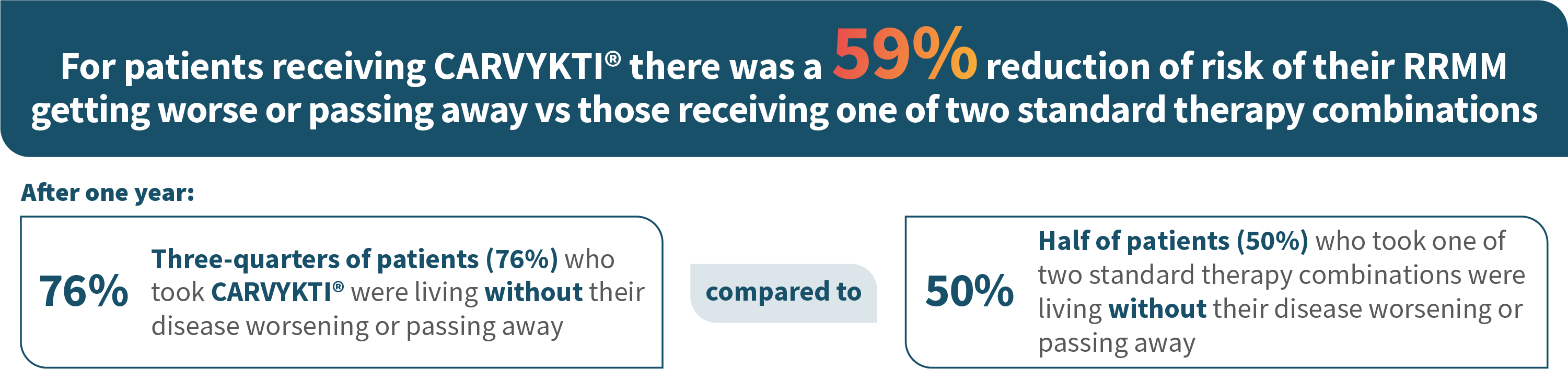
MM=multiple myeloma; RRMM=relapsed or refractory multiple myeloma.
CARVYKTI® was compared to patients receiving: daratumamab, pomalidomide, and dexamethasone or bortezomib, pomalidomide, and dexamethasone
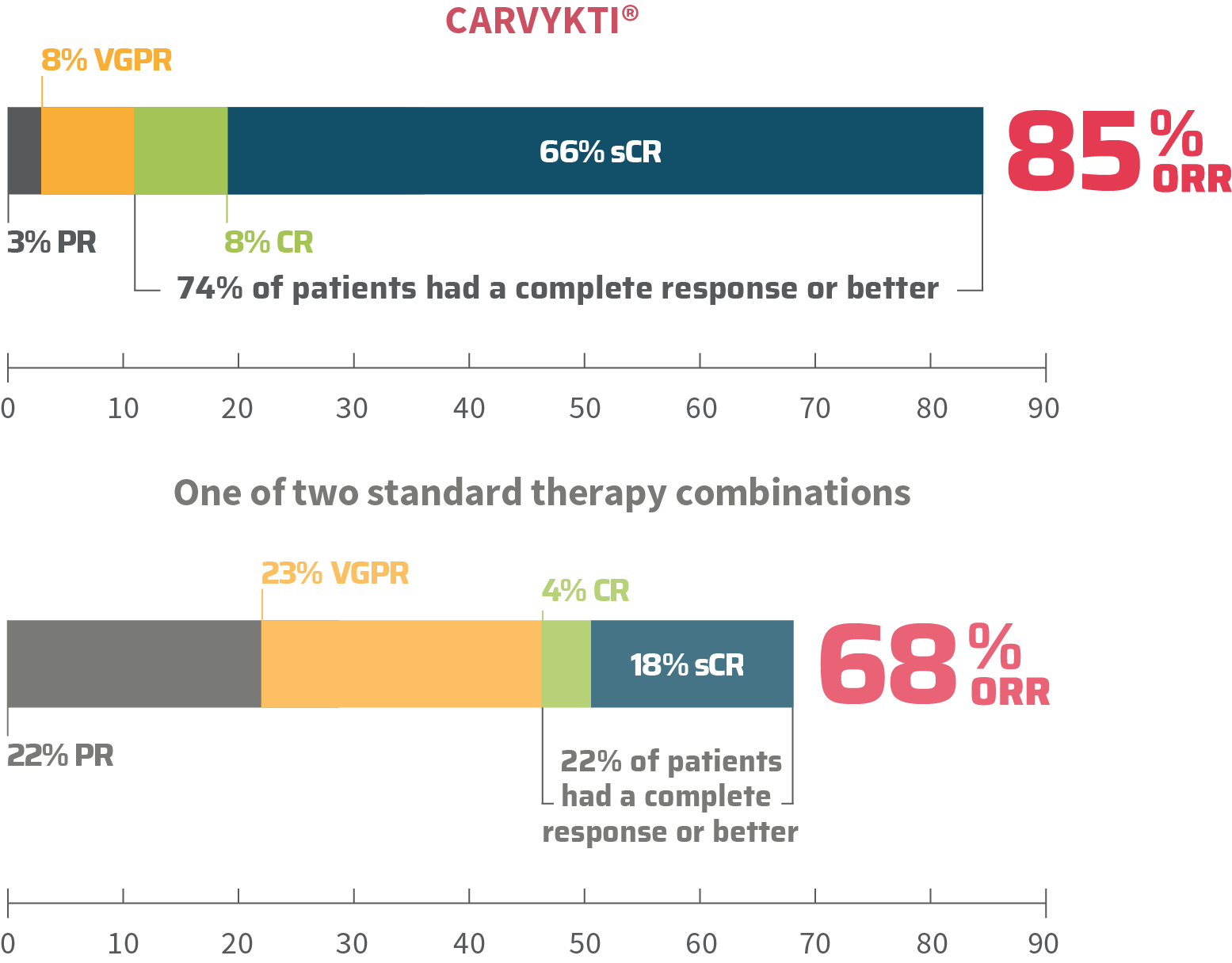
MM=multiple myeloma; RRMM=relapsed or refractory multiple myeloma.
CARVYKTI® was compared to patients receiving: daratumamab, pomalidomide, and dexamethasone or bortezomib, pomalidomide, and dexamethasone


*Median is the middle number in a group of numbers arranged from lowest to highest.
If you’re considering CARVYKTI®, you've already been treated with at least one treatment regimen that contained a proteasome inhibitor and an immunomodulatory agent, and you have not responded to lenalidomide.
In addition, your multiple myeloma has either returned or has stopped responding to treatment, and your doctor has determined that it's time to take the next appropriate step to help you manage this illness. Talk to your doctor to see if CARVYKTI® is right for you.
Fever (100.4°F or 38°C or higher)
Feeling confused, less alert, or disoriented
Difficulty speaking or slurred speech
Personality changes, including reduced ability to express emotions, being less talkative, disinterested in activities, and reduced facial expression
Fever
Confusion
Fever (100.4°F/38°C or higher), chills
CARVYKTI® may increase your risk of getting cancers, including certain types of blood cancers. Your healthcare provider should monitor you for this.